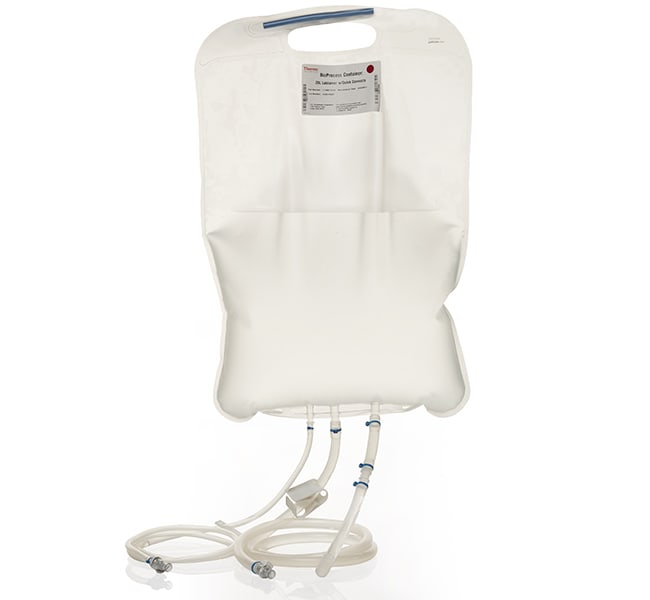
Aquapur purified water meets usp and multi compendial specification made for applications requiring usp ep grade purified water made via deionization reverse osmosis uv treatment and ultrafiltration aquapur meets specification including microbial specification through its 2 yr.
Water for injection usp ep.
Water for injection is classified for pharmaceutical purposes as an excipient in the production of parenteral preparations and in other pharmaceutical preparations where the endotoxin content must be verified see related usp ep and jp pharmacopeia for reference.
Water for injection quality wfi for cell culture meets usp ep monograph for water for injection at point of fill.
Water quality is monitored on a routine basis.
Requirements of the european pharmacopoeia 85 the european pharmacopoeia provides quality standards for the following grades of water.
Note water for injection is intended for use in the preparation of parenteral solutions.
It complies with both usp and ep monographs for water for injection packaged in bulk for commercial use solutions and standard administration supplies are available for injection and irrigation purposes.
Rmbio s water for injection wfi multi compendial grade is a terminally distilled sterile non pyrogenic preparation which contains no bacteriostat antimicrobial agent or added buffer.
Where used for the preparation of parenteral solutions subject to final sterilization use suitable means to minimize microbial growth or first render the water for injection sterile and thereafter protect it from microbial contamination.
Great deals on chemicals and chemical supplies.
Action levels in usp 1231 100cfu ml for purified water and 10cfu 100ml for water for injection are generally considered to represent a level above which the water is unfit for use.
86 water for injections 87 purified water 88 water for preparation of extracts 894 1.
It is intended to be used as a diluent in the preparation of parenteral products most typically for multi dose products that require repeated.
Water for injection wfi as defined by usp ep jp chp and ip monographs.
Potable water 90 potable water is not covered by a pharmacopoeial monograph but must comply with the regulations on.
The water is prepared by distillation and filtration 0 1 µm with no added substances.